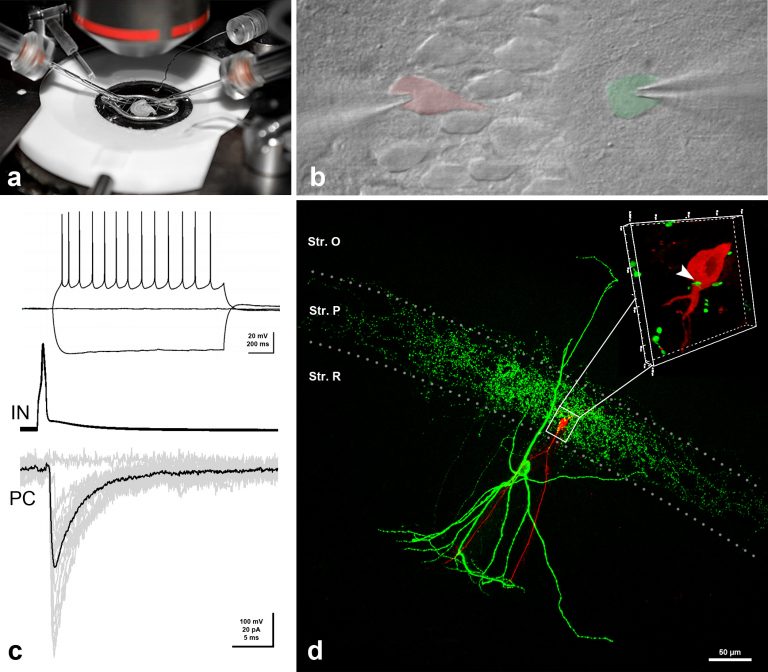
Patch-clamp electrophysiology
To investigate the functional significance of the signaling pathways, which we have uncovered in our molecular biology and anatomy experiments, we perform whole-cell patch-clamp recordings in acute brain slice preparations.
To prepare acute and organotypic slices, we use the latest Leica 1200S Vibratome. Our setup was built on the I-shaped Nikon FN1 upright microscope, and is positioned on a Supertech anti-vibration table. We use a water-immersion 40x CFI Apo objective with NA 0.8 and a working distance of 3.5 mm. Infrared DIC is utilized to enhance contrast in our samples and patching of selected neurons is aided by a Hamamatsu near infrared ½-inch CCD camera (C7500-51). An epifluorescence attachment also allows visualization of cells with genetically-encoded fluorescent markers, which help to identify and target specific cell types required for certain projects. Patch-clamp electrodes are pulled by a Sutter P-1000 horizontal pipette puller. Two Luigs & Newmann micromanipulator systems help us to carry out paired recordings or extracellular stimulation combined with single cell recordings. Whole-cell recordings are made in voltage- or current-clamp mode using an Axoclamp 2B amplifier from Axon Instruments. A second, almost identical setup is also available to us at the Light Microscopy Center located within the institute. This setup has a Nikon-C1 confocal attachment and with it we can monitor presynaptic calcium transients and postsynaptic responses simultaneously in our preparations. With the help of these electrophysiological setups, we aim to contribute to the understanding of how distinct endocannabinoid-related signaling pathways regulate certain forms of synaptic plasticity.