About us
The Laboratory of Neuronal Signaling has been established in 2011. The main focus of our group is to understand the cellular mechanisms underlying learning and memory processes.
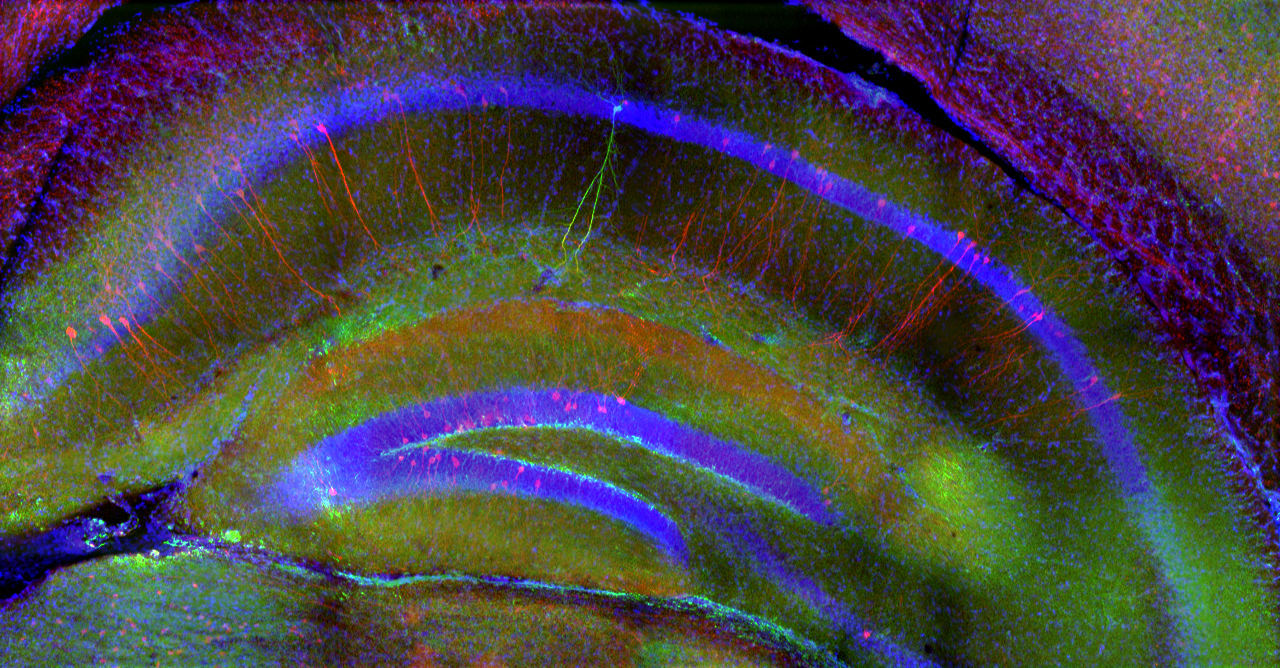
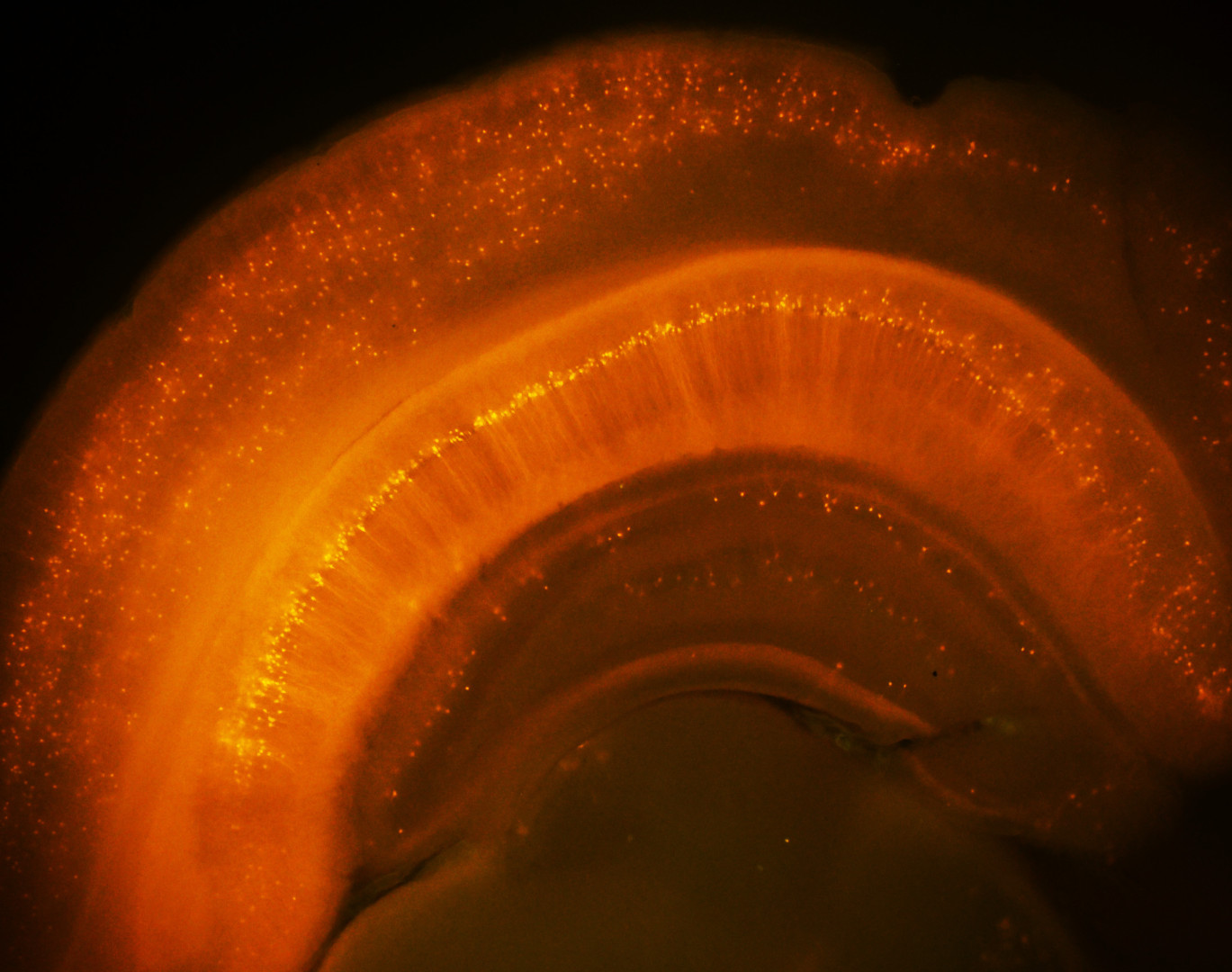
We study how the signal processing capabilities of individual neurons and the dynamics of their network in the hippocampus (a region of the brain that plays a central role in episodic memory processes) support spatial navigation, learning, and the storage and retrieval of memory traces. To this end, we investigate neuronal activity in the rodent hippocampus using cutting-edge in vivo and in vitro imaging and electrophysiological experiments.
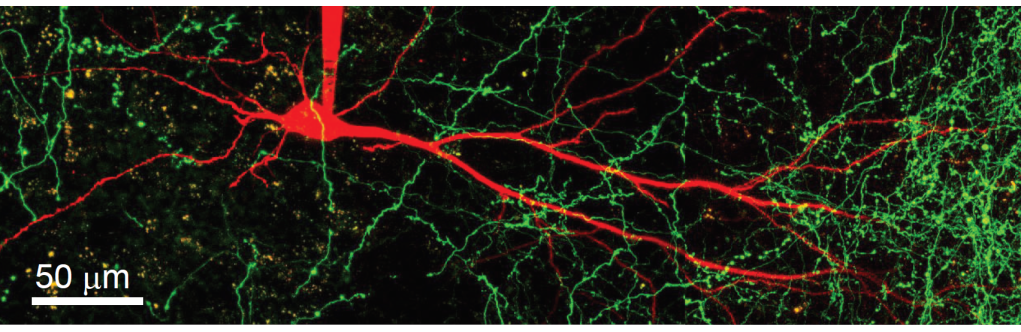
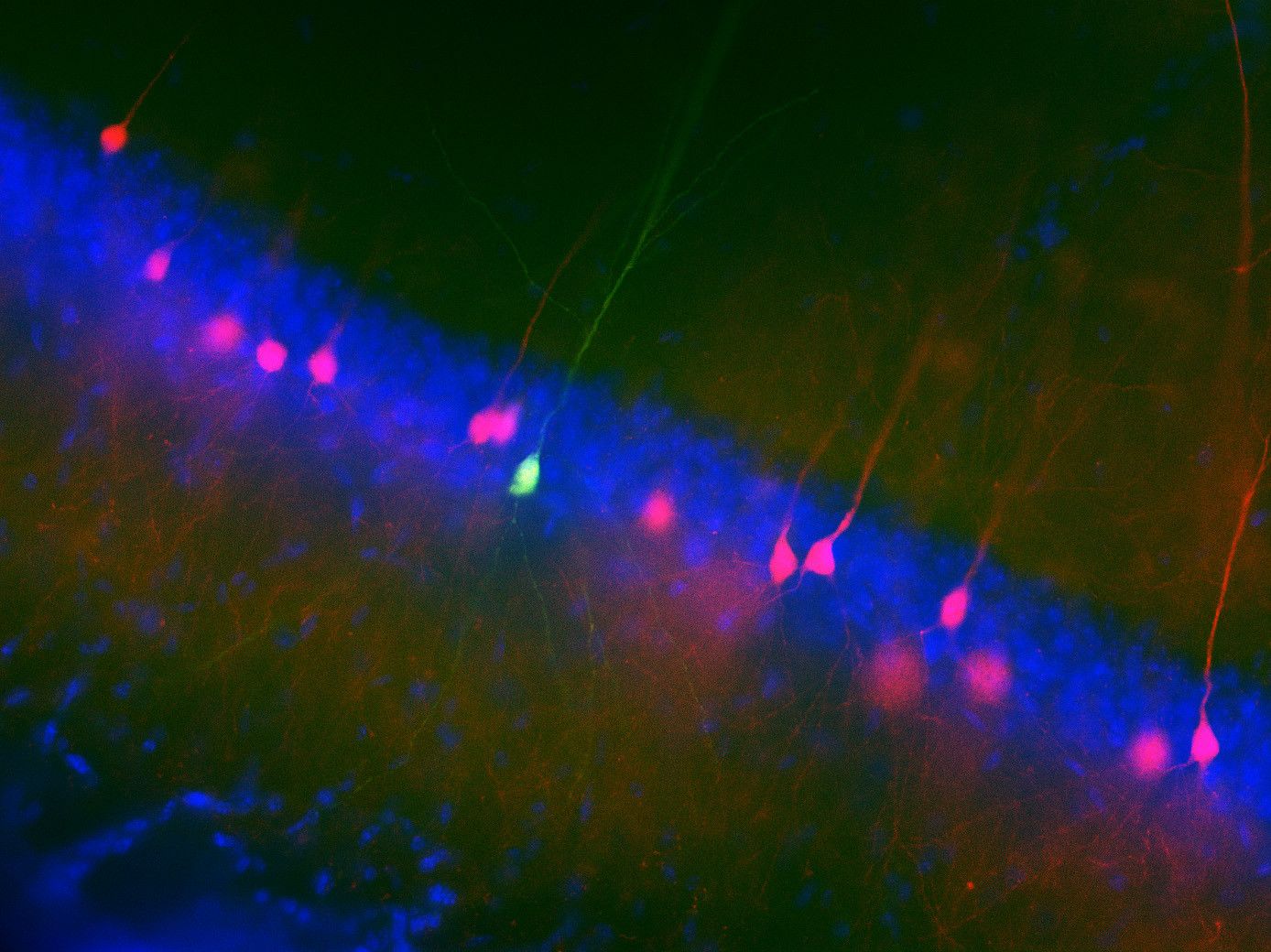
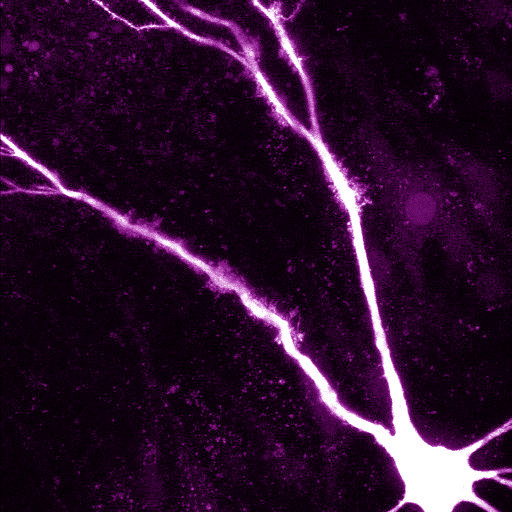
· One direction of our research is to understand the function of dendrites and dendritic spines, which are the subcellular information receiving and processing elements of neurons. The majority of mammalian neurons receive many thousands of synaptic inputs, which are located in the extensive dendritic tree. The passive and active electrical properties of the dendrites strongly influence the summation of the voltage responses produced by synaptic inputs. Understanding the molecular elements of dendritic function is therefore essential for understanding network computations related to cognitive function and behaviour.
The nonlinear properties of dendrites and dendritic spines are due, in part, to voltage-dependent ion channels expressed in their membranes. These ion channels can be activated upon the arrival of correlated input patterns and can alter the voltage response in the dendrite and shape the somatic output. This allows the cell to specifically recognise, process and respond to certain combinations of spatio-temporal input patterns. Moreover, activity-dependent regulation of dendritic ion channels makes the whole processing system dynamically variable.
Using a combination of in vitro multiphoton imaging and input stimulation methods and electrophysiological measurements in rodent brain slices, we aim to better understand the general as well as unique dendritic signal processing mechanisms in different types of hippocampal principal cells.
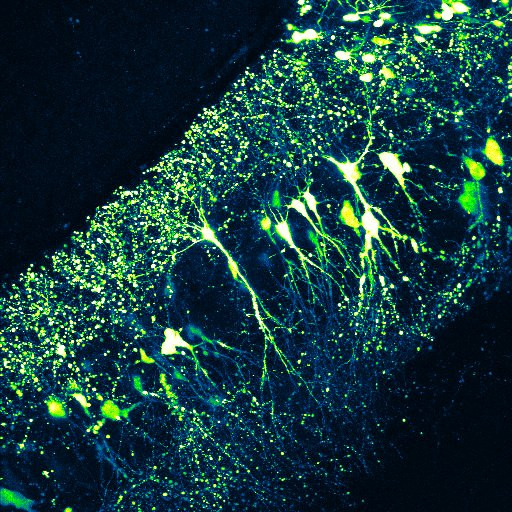
· In the other direction of our research, we monitor neuronal function using in vivo multiphoton imaging in awake mice performing various learning tasks. It has long been known that during spatial navigation, a subset of hippocampal pyramidal cells exhibits elevated activity at specific spatial locations in the environment, acting as "place cells”, and that the collective activity of these cells forms an internal map of the environment. However, relatively little is known about the short- and long-term cellular and subcellular mechanisms that support the emergence of spatially tuned cells in the different areas of the hippocampus, and how the activity dynamics of the neuronal network represent spatial and non-spatial elements of the environment that may be important for appropriate performance in the task.
In our experiments, head-restrained animals perform navigation-based tasks in virtual reality environments in which they have to learn where and according to what rules they can access reward. During the different phases of learning, neuronal activity is monitored using genetically expressed Ca2+ sensors, which (depending on the mode of expression) allow the activity of either a large cell population or subcellular elements (dendrites, axons) of individual neurons to be measured.
Our aim is to provide a coherent understanding of the mechanisms observed at different levels, from the function of individual neurons and their subcellular components to the information encoding dynamics of the network they form.
Funding supporting our research:
ERC Consolidator Grant
NAP3.0 program