The Mysterious GnRH
A good title is important not only for literature, but also for scientific publications. If it is too long or too complicated, it will hardly capture the attention of someone not deeply familiar with the topic. On the other hand, a well-chosen title can easily entice the reader to at least take a look at the summary.
It is likely that anyone browsing eLife - a journal widely read due to its high scientific standards and broad coverage of research topics - would have their eyes caught by the article titled “The cryptic gonadotropin-releasing hormone neuronal system”, published with Kata Skrapits as first author, even if it lies outside their own field of expertise. However, it is rather unlikely that the GnRH (gonadotropin-releasing hormone), described in the title as cryptic or mysterious, would be as familiar to readers with a general interest in the natural sciences as, for example, the hippocampus.
Fortunately, Erik Hrabovszky, the principal investigator and corresponding author of the study, can provide us with both interesting and important information.
– Let us perhaps begin with the fact that Roger Guillemin, working at the Salk Institute in San Diego, and Andrew Schally, working in New Orleans, isolated the GnRH decapeptide exactly fifty years ago, and proved its role in the hypothalamic regulation of reproduction.
– How big of a discovery was this back in 1971?
– This period was truly the golden age of endocrine research. Perhaps it is enough to say that both scientists received the 1977 Nobel Prize in Physiology or Medicine for their discoveries related to the production of brain peptide hormones. The medical application of GnRH and its later-developed effective analogues began almost immediately.
The historical significance of this discovery is well illustrated by the fact that the prestigious journal Journal of Neuroendocrinology plans to publish a special issue this year marking the 50th anniversary of GnRH’s discovery. Our group has been honored with an invitation to contribute a review summarizing the current knowledge on the human GnRH neuronal system. By fortunate coincidence, our article in eLife has also appeared in this jubilee year.
– My heartfelt congratulations both on the paper and the recognition!
But let’s return to the GnRH analogues and their medical applications. Could you give some examples?
– GnRH is a hypothalamic neurohormone that binds to its receptor located in the pituitary gland and stimulates the production of luteinizing hormone (LH) and follicle-stimulating hormone (FSH). Since these hormones control gamete and sex hormone production in the gonads, GnRH analogues can indirectly influence the function of the testes and ovaries.
Antagonists acting on the GnRH receptor (GnRHR1), such as cetrorelix, block LH production in the pituitary and thereby suspend testosterone production in the testes. This mechanism is successfully exploited in the medical treatment of testosterone-sensitive prostate cancer. The functional importance of the pulsatile pattern of GnRH secretion is highlighted by the fact that reduced testosterone production can also be achieved with GnRHR1 agonists (e.g. buserelin). In this case, the continuous presence of the stimulating analogue desensitizes pituitary GnRHR1 receptors, leading once again to reduced LH and testosterone production.
Of course, the medical applications are far broader. In gynecology, GnRH analogues are used in the therapy of endometriosis and in assisted reproductive technologies (ART) to treat infertility. In genetic disorders causing delayed puberty (hypogonadotropic hypogonadism), or in certain cases of centrally mediated infertility, therapy with a subcutaneously implanted GnRH pump providing pulsatile release can be highly effective.
– Your group is best known for research not only on GnRH, but also on another hypothalamic hormone, kisspeptin.
– Indeed, our international collaborations and recognition are primarily rooted in the study of hypothalamic kisspeptin and GnRH neurons. From our investigations on the human brain, we have published several review articles and book chapters.
Some of the central, long-standing questions in reproductive biology concern the mechanisms underlying the negative estrogen feedback present during most of the reproductive cycle, and the temporary positive feedback preceding ovulation. Also not well understood are the background mechanisms of the pulsatile release of GnRH and the onset of puberty. In all of these phenomena, kisspeptin neurons play a critical role. In fact, mutations in the genes encoding kisspeptin or its receptor in humans cause hypogonadotropic hypogonadism, a condition characterized by absent puberty and infertility.
– What were the immediate antecedents of your discovery now published in eLife?
– One special feature of GnRH neurons is that they differentiate outside the brain, in the nasal placode, and then, before birth, migrate along the nasal septum into the preoptic/hypothalamic brain regions. There, beginning after puberty, they carry out their lifelong secretory function by releasing pulses of the GnRH decapeptide.
In 2016, in collaboration with Paolo Giacobini’s group in Lille, France, we studied such migrating GnRH neurons. In Development, we described approximately 8,000 GnRH neurons that followed a previously unknown dorsal migratory route, targeting extrahypothalamic brain areas. We became curious about the fate of this neuronal population: could GnRH neurons be found in adult human brains outside the hypothalamus as well?
– Is it possible to know how many GnRH-producing neurons exist in the brain?
– The neuroendocrine regulation of reproduction is controlled—regardless of mammalian species—by only a few hundred to a few thousand GnRH neurons. These are located almost exclusively in the preoptic/hypothalamic area, and their axons project into the portal vasculature of the pituitary, releasing the GnRH neurohormone there.
The possible role of GnRH as a neurotransmitter had already been suggested earlier, since its receptor is widely present in the brain (for example in the hippocampus). However, the source of the GnRH peptide in those brain regions where no GnRH neurons or fibers are found has remained unknown.
It was therefore a major surprise when Kata and her colleagues, using stereological methods, identified as many as 150,000–250,000 “cryptic” GnRH neurons in adult human brain samples, specifically in the basal ganglia and the basal forebrain.
– Why had we not known about such a large number of neurons before?
– Extrahypothalamic GnRH neurons homologous to the human ones are not present in the brains of the most widely studied laboratory rodents. As a result, the possible non-reproductive roles of GnRH in the brain had not previously attracted attention. The hundreds of thousands of cells we observed in the adult human brain also make it highly unlikely that these extrahypothalamic GnRH neurons are identical to the much smaller nasal placode–derived GnRH population of the developing human brain.
– What other important observations did you make?
– The human tissue samples revealed that GnRH neuronal populations overlap significantly with known cholinergic cell groups. We detected GnRH immunoreactivity in a subpopulation of projection neurons of the nucleus basalis of Meynert, and most abundantly in cholinergic interneurons (ChINs) of the putamen.
– And what were you able to learn about the role of the extrahypothalamic GnRH neuronal system?
– The observations by Kata Skrapits and colleagues demonstrate that GnRH signaling in the brain is not limited to the control of reproduction. It also participates in peptidergic regulation of neuronal circuits in the striatal and basal forebrain regions.
– Were you able to confirm an actual neuronal signaling pathway?
– The greatest obstacle to clarifying the precise role of this peptidergic signaling is the lack of physiological animal models. As I mentioned, to date similar extrahypothalamic GnRH neurons have only been described in primate brains.
To explore the functional aspects, we established a newborn mouse model for electrophysiological experiments. In this system, Imre Farkas was able to detect a transient GnRH effect on cholinergic interneurons. The relevance of this model for the findings in the adult human brain, however, remains questionable.
– What experiments did you ultimately use to identify the target cells of GnRH action?
– We applied RNA-seq, a next-generation technique suitable for qualitative and quantitative detection of RNA, to neurons of the human putamen. These neurons were identified by size and isolated by laser capture microdissection (LCM).
From these results we know that GnRH produced in cholinergic interneurons of the putamen can act through the GNRHR1 autoreceptor to influence striatal functions. However, GNRHR1 mRNA was not present in the spiny projection neurons, which form the primary target cells of cholinergic interneurons.
– Your article has many co-authors, which is understandable given the wide range of techniques used. Surely all of them deserve to have their names attached to these results.
– Indeed. Both the members of our Laboratory of Reproductive Neurobiology and several external collaborators contributed to the work.
A significant portion of the laboratory and intellectual work is associated with Kata Skrapits. Using a gene gun, Szabolcs Takács was able to visualize the fine structural features of the dendritic trees of extrahypothalamic GnRH neurons, and he also demonstrated by in situ hybridization that the GnRH content of the putamen derives from the GNRH1 gene rather than GNRH2. The fact that these cells contain the genuine full-length GnRH decapeptide (10 amino acids) was confirmed by mass spectrometry measurements carried out by Blanka Tóth (BME).
Évi Rumpler, who recently obtained her PhD degree, contributed by performing control immunohistochemical experiments.
The RNA-seq experiments were methodologically the greatest challenge, as they required human brain samples with well-preserved tissue RNA. These samples were collected by myself together with Dr. András Matolcsy and Dr. Gergely Rácz at the 1st Department of Pathology and Experimental Cancer Research. The laser capture microdissection was performed by Balázs Göcz.
Since the feasibility of RNA-seq on post-mortem samples is not self-evident, we also had to develop a new methodology. This work, to which every colleague contributed considerable time and energy, was directed by Miklós Sárvári.
– You accomplished a huge amount of work! Are there any additional results you would highlight that we have not yet discussed?
– A by-product of the project was the transcriptomic characterization of human putamen spiny projection neurons and cholinergic interneurons, with the full RNA-seq dataset made publicly available. We were able to identify and quantify approximately 13,000 different mRNA transcripts per cell type. Based on the neurotransmitter and neurotransmitter receptor content of the studied neurons, we also constructed the so-called molecular connectome model of the two cell types.
– Where are your laboratory’s current research directions headed?
– Through methodological developments, we are now close to the goal of performing transcriptomic analyses of human neuronal cell types (such as GnRH and kisspeptin neurons) that are first identified by immunohistochemistry in autopsy tissue samples.
In rodents, we are currently investigating the molecular background of estrogen feedback in the brain. A manuscript led by Balázs Göcz as first author, in which we identified estrogen-dependent genes of kisspeptin neurons, is currently under review.
We are also applying the RNA-seq methodology to explore the functional background of clinically relevant problems such as infertility caused by high prolactin levels, caloric restriction, or polycystic ovary syndrome (PCOS).
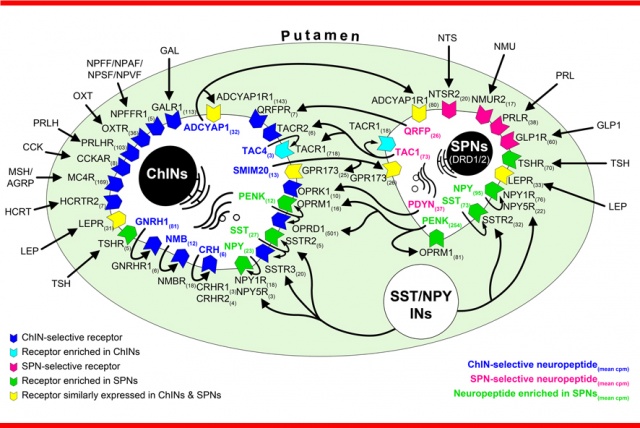
“Peptidergic connectome” of cholinergic interneurons and spiny projection neurons in the human putamen






