On thyroid hormone migration and ways of cognition
Scientists will never be surprised to find themselves out of a job because all the questions have been answered and there is nothing more to discover. The work of the teams of Balázs Gereben and Csaba Fekete, published in the journal eLife, has given a new and correct answer to an old question, and the discovery can also be used in translational medicine.
Technically, it was known since antiquity, first named in connection with the alchemists in the Middle Ages, Newton built his own by himself. What is it? The laboratory, which, especially in the natural sciences, is the primary site of research, and can be as varied as the research itself. Specially equipped laboratories are needed not only to carry out experiments but also to repeat them under strictly identical conditions. Indeed, if the repetition of an experiment does not give the same result, it is discredited and, however sensational, cannot be published.
The laboratories of a research institute often have to carry out 'preparatory work' to help the planned experiment. They do the same as many excellent craftsmen or artists from ancient times to the present day. They could make their own tools, quarried stones for painting, to design chairs and stands to make a sculpture, paintings, frescos, or even, a basilica. Similarly, some of today's researchers put together a microscope that never existed before (three have even won Nobel Prizes for it), and others need a model of an animal to create one.
An excellent example is the patented "Thyroid Hormone Action Indicator" (THAI) transgenic mouse model, which is used to measure the action of thyroid hormone (TH). This model made it possible for the research teams led by Balázs Gereben and Csaba Fekete together with their American colleagues, to make an important discovery.
Many people know that the thyroid gland is a very important organ, or that goiter was a common serious disease in iodine-deficient areas, which has been virtually eliminated in countries that have introduced the use of iodized salt. However, Balázs Gereben and Csaba Fekete are among the few people who know much more about the thyroid gland than that. Their discovery, published in the scientific journal eLife, not only explains a long-debated question but also has applications in translational medicine.
(At the request of Balázs Gereben and Csaba Fekete, we have not named the person who answered the respective question, because the answers reflect their joint opinions.)
- What should everyone know to understand your discovery and its significance?
- Thyroxine (T4) and triiodothyronine (T3), produced by the thyroid gland, are hormones that affect the development and function of most cells in our body, so their adequate levels are critical for maintaining normal functioning of the whole body. T4, produced in significantly higher amounts, is a prohormone that cannot bind to a receptor. This prohormone is converted by enzymes cleaving iodine into the active T3, which exerts its biological effect by binding to thyroid hormone receptors.
- What are the typical diseases of the thyroid gland and has their prevalence changed?
- The most common is hypothyroidism, i.e. a deficiency of thyroid hormones. This disease affects hundreds of millions of patients worldwide, e.g. 30 million people in the USA alone! Some patients in this group have had to have their thyroid gland removed due to cancer and therefore need hormone replacement for the rest of their lives. Today, the number of diagnosed thyroid tumors is increasing. This is partly due to advances in diagnostic techniques and partly due to the delay in detecting many tumors today because of the lack of testing during the Covid era.
In a significant number of other patients, autoimmune inflammatory thyroid diseases lead to thyroid hormone deficiency. In the past, hypothyroidism was also caused by iodine deficiency, which fortunately has now almost disappeared with the widespread use of iodized salt.
- Could there be another cause of hypothyroidism?
- A less common cause may be congenital thyroid hormone deficiency due to genetic causes. Such diseases used to cause severe mental retardation, but thanks to screening in newborns, this is now largely preventable by thyroid hormone supplementation.
- What should we know to understand the purpose of your research?
- Thyroid hormone levels in the bloodstream are relatively stable, while turbulent and rapid changes in TH levels are observed in different tissues and cell types. This is also necessary to meet the rapidly changing spatial and temporal demand for TH in tissues. However, it is not known how thyroid hormones are able to produce tissue-specific effects in addition to the stable circulating blood levels and the general effects mentioned above.
- And what do we now know "for sure" about this?
It is now clear that this is made possible by a very complex tissue- and in many cases cell-type specific molecular regulatory system that regulates the metabolism and effects of TH in tissues. This local regulatory system is of particular importance in the brain. Therefore, it is our common goal to elucidate the mechanisms involved in the cell-type-specific regulation of TH action in the brain, as well as in other tissues of our body.
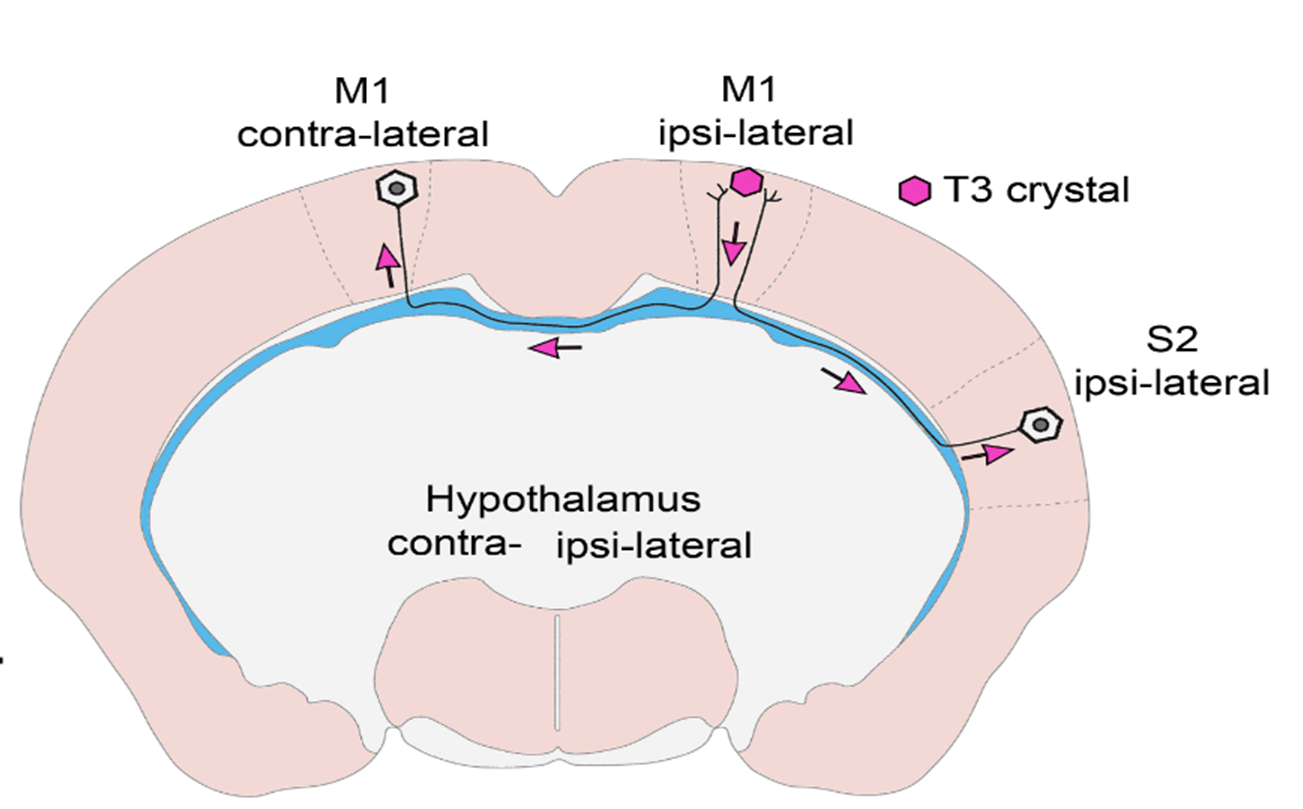
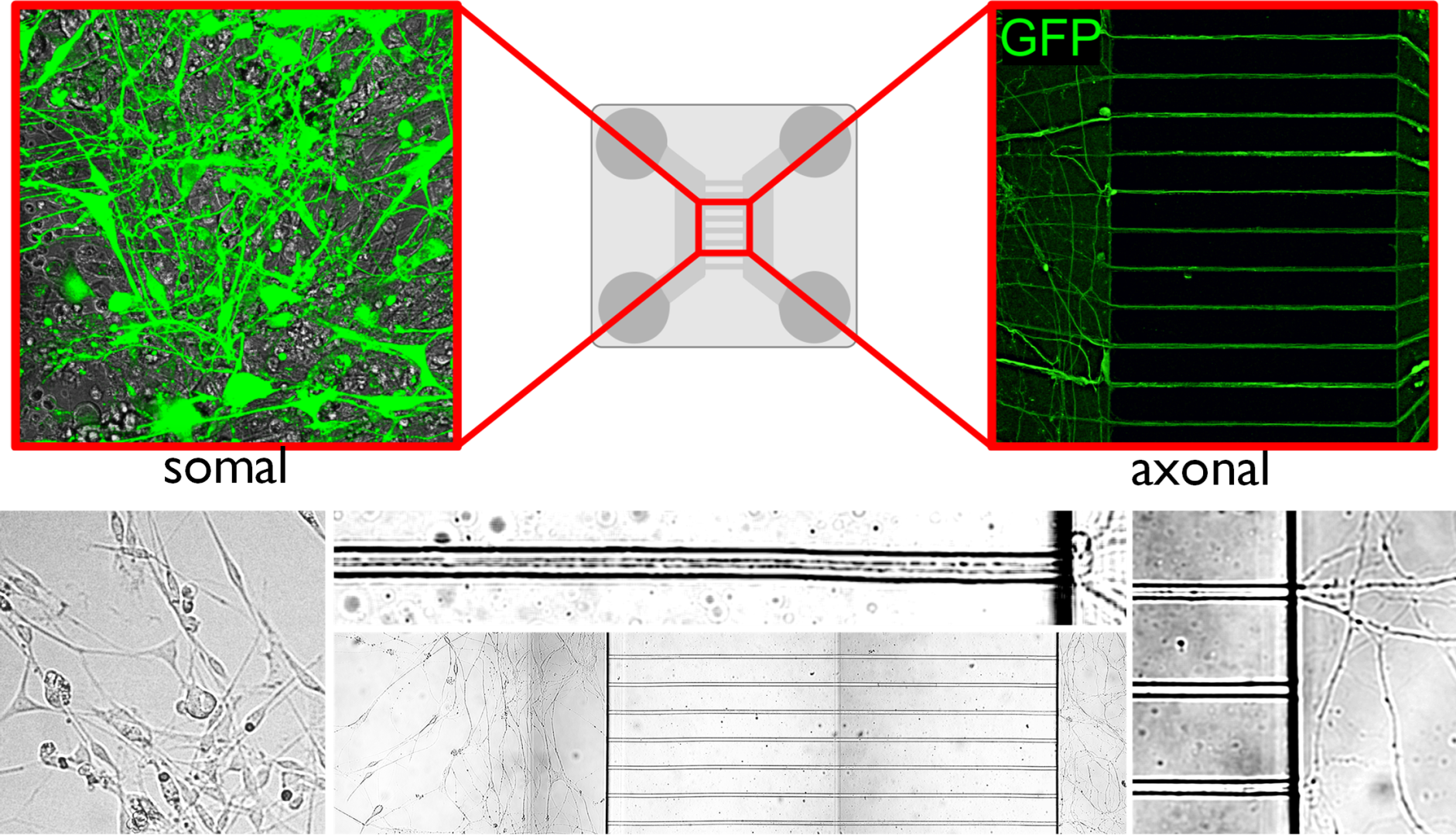
- What have you found out so far and what is the new type of regulatory mechanism you have identified?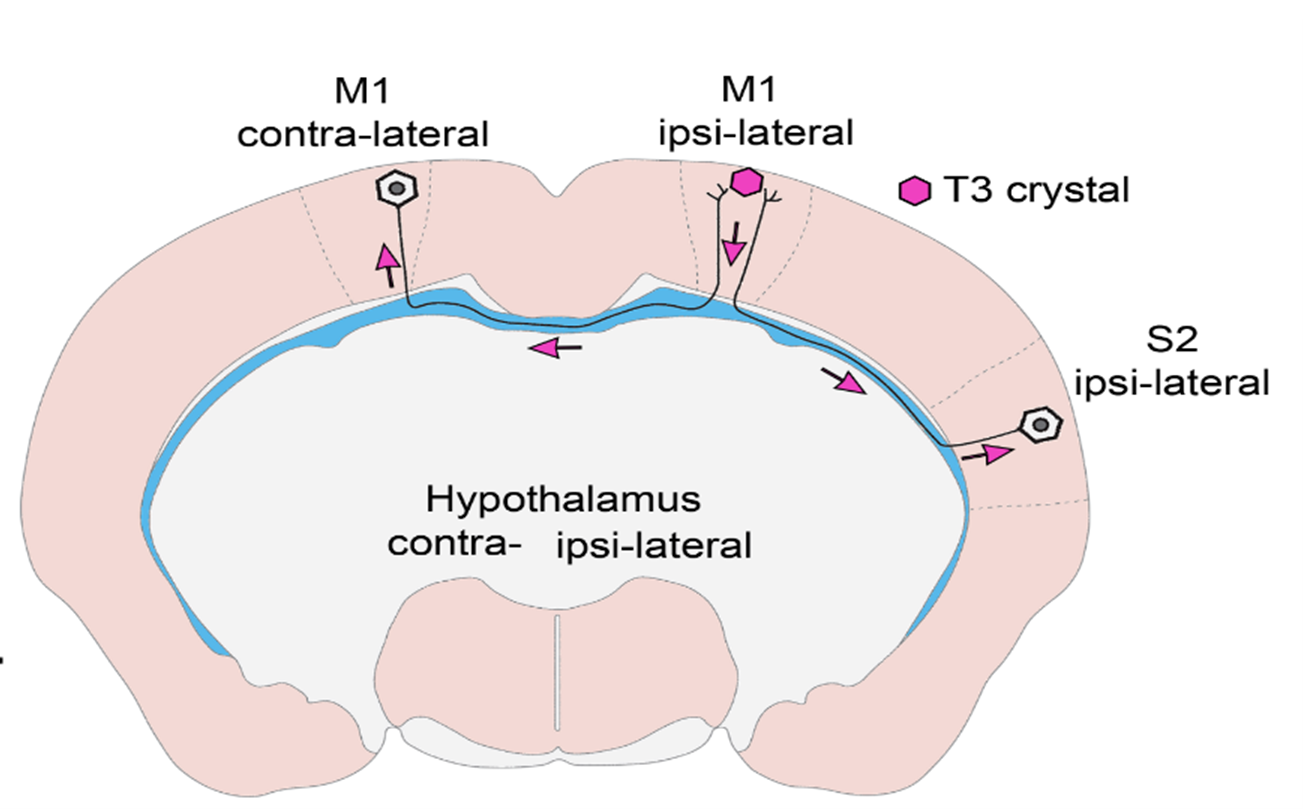
- In vivo and in vitro experiments have shown that T3 travels retrograde, backward along neuronal axons to reach their nucleus in vesicles. The vesicles can protect the hormone from the T3-degrading enzyme that is present in high levels in nerve cells. In this way, T3 generated in the cerebral cortex can be transported to the other side of the cortex via the long axons connecting the hemispheres, so that distant brain regions can regulate each other's TH-dependent cell activity! This finding also helps to answer some long-standing unresolved questions in the field. We have shown that the hypothalamic-pituitary-thyroid (HPT) axis-regulating neuronal group, the hypophysiotropic TRH neurons of the hypothalamus, also receive T3 input from the peripheral circulation and from the tanycytes, the TH-activating cells of the hypothalamus.
- Why is this so important?
- It is essential because it is the basis of the negative feedback that ensures stable blood thyroid hormone levels! This in turn has an effect on the whole body, as the TH-induced negative feedback regulation of TRH neurons is a key regulatory mechanism of the HPT axis.
- Did you specifically design your experiments to explain this negative feedback, or did you "get lucky" and immediately understand the significance of what you measured-found?
- Our work was originally started to better understand feedback. This region of the hypothalamus has long been of particular interest to us because it is the region, where, even if not near each other, the TH-regulated TRH neurons and the thyroid hormone-activating tanicytes are located. However, the axons of TRH cells are located near the extensions of the tanycytes, raising the possibility that thyroid hormone transport within these axons may be the key to answering this question. In interpreting our experimental results, we realized that a more general phenomenon underlies our findings, and extended our studies to the cerebral cortex. It turns out that this mechanism works there too!
- Your experiments were carried out in a rodent model, but in addition to the value of your findings in basic science, they also have a translational, medical significance.
- At present, thyroid hormone replacement is based almost exclusively on T4 monotherapy. This helps many people, but it is not a satisfactory solution for 10-20% of the huge group of patients mentioned. Many treated patients in the mentioned subgroup complain of symptoms typical for brain hypothyroidism, despite the fact that their TSH levels are within the normal range as a result of T4 treatment. Despite the importance of the problem, it has been completely ignored for decades, and only recently has attention begun to focus on how to improve the treatment of these patients.
- What might this mean in practice?
-Our mechanism, which protects T3 transported in axons from the thyroid hormone-degrading enzyme also produced in neurons, resolves a paradox. Some patients who respond poorly to T4 monotherapy for hypothyroidism feel better on T4+T3 combination therapy despite the very significant T3-degrading capacity in the brain. It is now known, that T3 is able to exert its effects due to its protected migration, and our present data contribute to our understanding of the mechanism of action of T4+T3 combination therapy as an alternative.
It is also promising that our new studies which are in the advanced phase and were not reported in the publication will bring us even closer to understanding the symptoms of this patient group.
: Axonal T3 uptake and transport can trigger thyroid hormone signaling in the brain. May 19, 2023 https://doi.org/10.7554/eLife.82683
Agnes Kittel