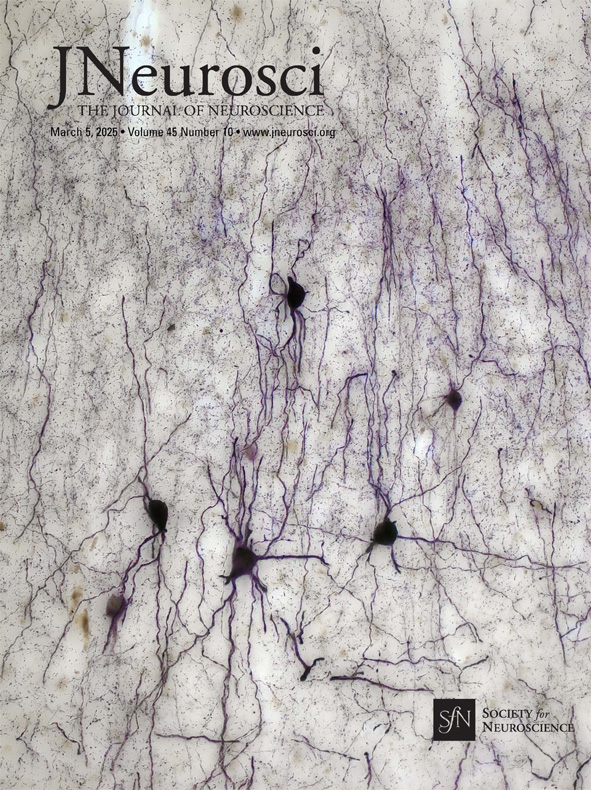
Another step towards understanding the human hippocampus and its diseases
The hippocampus is not only known to be crucial for learning and memory processes, but also for neurological and psychiatric disorders and diseases such as schizophrenia, epilepsy and Alzheimer's disease. Nevertheless, our knowledge of the human hippocampus is still incomplete. Taken as a whole, the findings of Gábor Nyiri's group, published in the Journal of Neuroscience, are fundamental and filling a gap - and some of them are beautiful. That's why the journal's editorial board chose one of their micrographs as the cover photo.
The study of the hippocampus has been a major and successful project of our institute for three decades. It was the research area of György Buzsáki,Tamás Freund (now the President of the MTA, then our Director) and Peter Somogyi, who were awarded the first Brain Prize in 2013 and the entire research work of John O'Keefe, who together with his students May-Britt and Edvard Moser won the Nobel Prize in Medicine in 2014. The list of significant discoveries could go on and on - but they are not primarily about the human hippocampus. This would be all the more important because computer models and artificial intelligence can only help to understand brain function, and in particular the hippocampal neural networks, if reliable basic data are available. And this is precisely what we have not yet been able to say about the human brain.
Our questions, starting with what was the reason for this "lag" and what made possible and timely the fundamental and gap-filling studies that earned the recognition of the editors of the Journal of Neuroscience, are answered by Virág Takács, one of the first authors of the article and a senior researcher in Gábor Nyiri's group.
- To begin with, there have been several studies on the morphology, location and changes in the morphology of interneurons in human samples that have been studied in various neurological diseases. However, an accurate estimate of their total number has not yet been made.
- How could this happen?
- There are several reasons for this, perhaps the most important of which is that the high quality immunostaining required for such studies can only be performed on short, in our own studies up to 3.5 hours post mortem, and properly perfused samples. Second, the strict rules of stereological studies require systematic stereological sampling, for which tissue preservation, i.e. fixation, of the entire hippocampus must be uniformly good. This hard condition is usually difficult to meet.
- But you did it!
- We are also grateful for it, especially to Zsófia Maglóczky from KOKI and the Human Brain Tissue Laboratory, and to the pathologists of the Szent Borbála Hospital in Tatabánya, especially Dr. Peter Gombás! Thanks to them, we finally managed to process four high quality samples.
- I suppose the difficulties and challenges of the work did not end there. . .
- A further challenge of the research was the workload of the task. The entire hippocampus had to be sectioned and then the sampled sections plotted using software. We needed 94 sections for cell counting alone, and more for volume measurement. Sometimes it took a whole day just to mark the cells in a single section. To generate the electron microscopy data, we embedded samples from all layers of all hippocampal regions (15 layers in total), several different samples and immunostaining stains, from which serial sections were taken. From the scanning electron microscope section sequences (a single sequence represents more than 50 sections), we visualized immunolabeled axon terminals over a large area using three-dimensional reconstruction. This required many hours of work.
- Even to a layman, it seems like a lot of work. How did you organise the process?
- We divided the tasks. Papp Peti, Zsoldos Tamás, Zichó Krisztián and myself did most of the light microscope drawing, Zsuzsi Bardóczi and I worked together on the electron microscope work, while Áron Orosz, Papp Peti and I were mainly involved in the data evaluation.
- What determined how, what data should be collected and in what format to make them suitable for the creation of computer models?
- We undertook this project in the framework of the European Union Human Brain Project, so we knew from the very beginning exactly what data we needed to provide and in what format. The total number of different inhibitory neurons and synapses per subregion and per layer is essential information to create an anatomically accurate network model. All the raw data are reported in the supplementary tables in this article and we are confident that they will be incorporated into the latest models soon.
- Has a computer model of the human hippocampus been made recently?
- Gandolfi and colleagues published their model of the CA1 region of the human hippocampus in 2023. In the absence of human data, the authors relied heavily on values obtained for rodent interneurons, scaling them to the known human pyramidal number.
- Based on your results, how close do you think they were to the real thing?
- Significant differences were found between human and rodent species in the proportion of interneurons containing the three main inhibitory cell groups, parvalbumin, somatostatin and calretinin! For example, while in rats the largest group is made up of parvalbumin-positive interneurons, in our human samples we found five times as many calretinin-positive cells as parvalbumin-positive interneurons. This suggests that fine-tuning of existing models is essential and that our data may contribute to a more accurate, human-specific network representation.
- Hats off also to your brave undertaking and the amount of work you have done, but the values obtained, the total cell count and the total number of inhibitory synapses, are not absolute but estimated values. What has prevented you from getting an accurate figure and how close is this estimate to reality?
- The size of the hippocampus itself shows a huge individual variance, so there is naturally a large variation in cell numbers. Since, due to the limitations mentioned above, we were only able to analyse the hippocampus of four subjects, our data set can be interpreted primarily as a first, approximate estimate.
- In your huge work, what proportion of hippocampi have you analysed in detail in relation to their total volume, and what is the basis for claiming that the data are accurate?
- Following the stereological rules, on average about one tenth of the total tissue volume was used for different study purposes. That additional sampling would not significantly affect the accuracy of the data was statistically verified by separate sampling. The remaining sections are frozen and await future projects.
- How large human hippocampal samples were obtained?
- Whole hippocampi were obtained and sectioned with a vibratome. We then systematically sampled from many sites. This was also necessary because the hippocampus is characterised by slightly different connections and functions along its longitudinal axis, so the density of different cell types is not necessarily the same throughout its volume.
- Why couldn't the tissue be made transparent, avoiding tissue loss due to incisions, and thus perform the labelling?
- Although making the tissue transparent could have been an alternative approach, it would have been impractical to do so for human post-mortem specimens for several reasons. The purification would also wash out some of the cell-identifying proteins, full tissue penetration of antibodies would be almost impossible, and the autofluorescence of pigment granules called lipofuscin that accumulate in human cells would cause the background of immunostaining to be so high that it would make detection of specific labeling difficult. Therefore, the conventional section-based method and light microscopy detection of the label were chosen.
- Were there any work steps or procedures that you modified based on your experimental experience?
- For the first sample, we noticed that the quality of the immunostaining deteriorates when tissue blocks are stored in acidified phosphate buffer for a longer period of time (weeks). Therefore, we tried to section the whole hippocampus as soon as possible and used the sections stored in 30% sucrose, retrieved from the freezer, for the experiments.
We also observed that it is conceivable that the use of artificial intelligence, i.e. automatic detection, could be applied to the images taken with the slide-scanner for the parvalbumin-stained cell bodies. However, this is not feasible for somatostatin and calretinin scanners, as their staining is often faint.
We have started to develop a synapse detection software that we will use for our electron microscopy studies.
- We have learned a lot about the technical background of your experiments, the difficulties you have overcome, but also the limitations. It is certainly no coincidence that you have chosen parvalbumin, somatostatin and calretinin interneurons for your studies. In which neurological/psychiatric diseases do they play an important role, and what questions do you think a model built using the new data could answer most quickly?
- It is known that the density of parvalbumin-positive hippocampal interneurons is reduced in schizophrenia, and Zsófia Maglóczky's group showed that there are fewer calretinin-expressing interneurons in epileptic patients. Animal experiments suggest that dysfunction of somatostatin-positive interneurons may also play a role in the network changes induced by epilepsy. These latter two interneuron types are particularly sensitive to vascular occlusion, and there is also evidence that Alzheimer's disease may affect different interneuron populations differently.
To better understand both the development and the mechanisms of the aforementioned diseases mentioned in the short list above, anatomically accurate models based on realistic human interneuron data are needed.
Our work has contributed to this.